
A2.832 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis and 6.439 grams of co2 and 2.636 grams of h2o are produced. in a separate experiment, the molar mass is found to be 116.2 g/mol. determine the empirical formula and the molecular formula of the organic compound. enter the elements in the order c, h, o empirical formula = molecular formula =

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2
You know the right answer?
A2.832 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis a...
Questions

English, 03.10.2021 22:20

Mathematics, 03.10.2021 22:20




English, 03.10.2021 22:20

Chemistry, 03.10.2021 22:20

English, 03.10.2021 22:20

World Languages, 03.10.2021 22:20







Mathematics, 03.10.2021 22:20

Arts, 03.10.2021 22:20



History, 03.10.2021 22:20

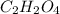 and
and 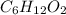 respectively.
respectively.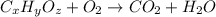
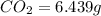
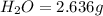
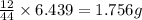 of carbon will be contained.
of carbon will be contained.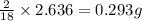 of hydrogen will be contained.
of hydrogen will be contained.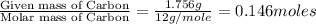
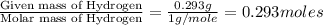
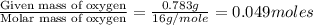
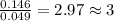
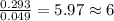
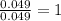
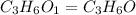
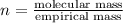
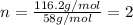
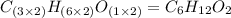
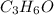 and
and 

