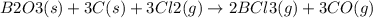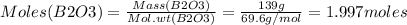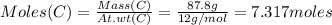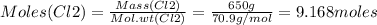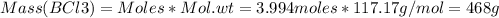Consider the reaction of diboron trioxide with carbon and chlorine. b2o3 (s) + 3c (s) + 3cl2 (g) 2bcl3 (g) + 3co (g) determine the limiting reactant in a mixture containing 139 g of b2o3, 87.8 g of c, and 650 g of cl2. calculate the maximum mass (in grams) of boron trichloride, bcl3, that can be produced in the reaction. the limiting reactant is: b2o3 cl2 c amount of bcl3 formed = g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
Consider the reaction of diboron trioxide with carbon and chlorine. b2o3 (s) + 3c (s) + 3cl2 (g) 2bc...
Questions














Mathematics, 17.01.2021 01:00




Mathematics, 17.01.2021 01:00


Mathematics, 17.01.2021 01:00