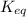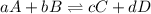Consider the general reversible reaction.
aa + bb
c + dd
what is the equilibrium c...


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1


Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
Questions



English, 13.02.2020 18:20


Arts, 13.02.2020 18:20








Arts, 13.02.2020 18:20



Mathematics, 13.02.2020 18:20

Mathematics, 13.02.2020 18:20



![K=\frac{[D]^d[C]^c}{[B]^b[A]^a}](/tpl/images/0173/5628/7e20b.png)