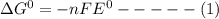Consider the following half reaction in which nitrate is reduced to nitrite: no3– 2h 2e– → no2– h2o. the reaction has a δg°' value of –81 kj/mol. the negative value of δg°' results from the very e°' value of the redox couple no3–/no2–, and it reflects the strong tendency of nitrate to electrons. choose one: a. positive / donateb. negative/ acceptc. negative / donated. positive / accept

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

You know the right answer?
Consider the following half reaction in which nitrate is reduced to nitrite: no3– 2h 2e– → no2– h2o...
Questions

Mathematics, 12.12.2021 05:20


Biology, 12.12.2021 05:20

English, 12.12.2021 05:20


Mathematics, 12.12.2021 05:20

English, 12.12.2021 05:20


History, 12.12.2021 05:20


English, 12.12.2021 05:20


History, 12.12.2021 05:20

History, 12.12.2021 05:20

Mathematics, 12.12.2021 05:20