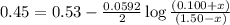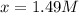Chemistry, 09.08.2019 21:20 thatcurlysophia
77. a voltaic cell consists of a zn/zn2+ half-cell and a ni/ni2+ half-cell at 25°c. the initial concentrations of ni2+ and zn2+ are 1.50 m and 0.100 m, respectively. a. what is the initial cell potential? b. what is the cell potential when the concentration of ni2+ has fallen to 0.500 m? c. what are the concentrations of ni2+ and zn2+ when the cell potential falls to 0.45 v?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Write skeleton equations for the following reactions c. aluminum(s)+copper(i) chloride(aq) > aluminum chloride(aq)+copper(s)
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
77. a voltaic cell consists of a zn/zn2+ half-cell and a ni/ni2+ half-cell at 25°c. the initial conc...
Questions


Mathematics, 28.07.2019 11:20


Mathematics, 28.07.2019 11:20

History, 28.07.2019 11:20



Mathematics, 28.07.2019 11:20

Spanish, 28.07.2019 11:20


History, 28.07.2019 11:20

Mathematics, 28.07.2019 11:30

Computers and Technology, 28.07.2019 11:30




Mathematics, 28.07.2019 11:30

History, 28.07.2019 11:30


Mathematics, 28.07.2019 11:30

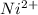 has fallen to 0.500 M is, 0.52 V
has fallen to 0.500 M is, 0.52 V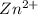 when the cell potential falls to 0.45 V are, 0.01 M and 1.59 M
when the cell potential falls to 0.45 V are, 0.01 M and 1.59 M![E^0_{[Ni^{2+}/Ni]}=-0.23V](/tpl/images/0173/8654/be6be.png)
![E^0_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0173/8654/4cd18.png)
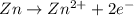
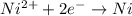
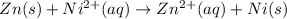
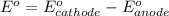
![E^o=E^o_{[Ni^{2+}/Ni]}-E^o_{[Zn^{2+}/Zn]}](/tpl/images/0173/8654/7d468.png)
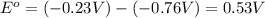
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}]}{[Ni^{2+}]}](/tpl/images/0173/8654/c02a9.png)
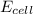 = emf of the cell = ?
= emf of the cell = ?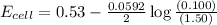
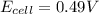
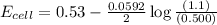
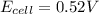
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}+x]}{[Ni^{2+}-x]}](/tpl/images/0173/8654/e92ff.png)