

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
For the reaction 2 a ⇌ b, the equilibrium concentrations are as follows: [a] = 0.056 mand [b] = 0.1...
Questions

Mathematics, 28.10.2020 04:10





Mathematics, 28.10.2020 04:10

Physics, 28.10.2020 04:10

History, 28.10.2020 04:10

History, 28.10.2020 04:10


Mathematics, 28.10.2020 04:10

Advanced Placement (AP), 28.10.2020 04:10


Mathematics, 28.10.2020 04:10



Medicine, 28.10.2020 04:10


Mathematics, 28.10.2020 04:10

Chemistry, 28.10.2020 04:10

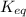 .
.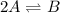
![K_{eq} = \frac{[B]}{[A]^{2}}](/tpl/images/0173/8965/36e57.png)
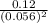
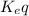 ) for the reaction is 38.
) for the reaction is 38.

