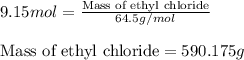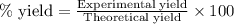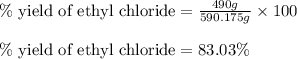Chemistry, 09.08.2019 23:20 4804397217
The reaction of ethane gas (c2h6) with chlorine gas (cl2) produces c2h5cl as its main product. calculate the percent yield of c2h5cl if the reaction of 300 g of ethane with 650 g of chlorine produced 490 g of c2h5cl .

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1


Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
The reaction of ethane gas (c2h6) with chlorine gas (cl2) produces c2h5cl as its main product. calcu...
Questions

Arts, 15.12.2020 21:10




Spanish, 15.12.2020 21:10


Mathematics, 15.12.2020 21:10


History, 15.12.2020 21:10



Biology, 15.12.2020 21:10

Biology, 15.12.2020 21:10


Mathematics, 15.12.2020 21:10

Mathematics, 15.12.2020 21:10


Mathematics, 15.12.2020 21:10

Biology, 15.12.2020 21:10

Mathematics, 15.12.2020 21:10

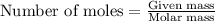 ....(1)
....(1)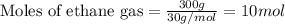
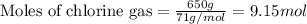
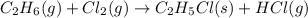
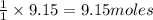 of ethane gas.
of ethane gas.