
Chemistry, 10.08.2019 00:10 makennskyee1198
If the reaction n2 (g) + 3 h2 (g) --> 2 nh3 (g) has the concentrations 1.1 m for nitrogen, 0.75 m for hydrogen and 0.25 m for ammonia gas, what is the kc? show all work. does this mean that there are more reactants or products at equilibrium? explain how you determined that.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 12:30
0.070g of hydride of carbon occupies 56cm^3 at s.t.p when vaporized and contained 14.29% by mass of hydrogen.what is the formula for the hydrocarbon
Answers: 1
You know the right answer?
If the reaction n2 (g) + 3 h2 (g) --> 2 nh3 (g) has the concentrations 1.1 m for nitrogen, 0.75...
Questions

Mathematics, 26.09.2019 18:00

History, 26.09.2019 18:00

Mathematics, 26.09.2019 18:00

Mathematics, 26.09.2019 18:00

Physics, 26.09.2019 18:00


Mathematics, 26.09.2019 18:00


Physics, 26.09.2019 18:00

Physics, 26.09.2019 18:00

Mathematics, 26.09.2019 18:00

Mathematics, 26.09.2019 18:00


History, 26.09.2019 18:00

Biology, 26.09.2019 18:00

Biology, 26.09.2019 18:00

Advanced Placement (AP), 26.09.2019 18:00

Mathematics, 26.09.2019 18:00

Geography, 26.09.2019 18:00

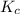 is 0.136 and is reactant favored.
is 0.136 and is reactant favored.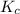
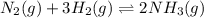
![K_{c}=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0173/9631/f3b94.png)
![[NH_3]=0.25M](/tpl/images/0173/9631/306b2.png)
![[H_2]=0.75M](/tpl/images/0173/9631/5c336.png)
![[N_2]=1.1M](/tpl/images/0173/9631/4c5ce.png)
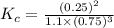
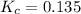
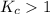 ; the reaction is product favored.When
; the reaction is product favored.When 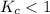 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 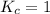 ; the reaction is in equilibrium.
; the reaction is in equilibrium.


