
Suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with iron(ii) chloride, which would react with silver nitrate solution like this: fecl2 (aq) + 2agno3 (aq) → 2agcl (s) + feno32 (aq) the chemist adds 14.0m m silver nitrate solution to the sample until silver chloride stops forming. she then washes, dries, and weighs the precipitate. she finds she has collected 6.9mg of silver chloride. calculate the concentration of iron(ii) chloride contaminant in the original groundwater sample. be sure your answer has the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 10:30
Amethod of separation that employs a system with two phases of matter, a mobile phase and a stationary phase, is called
Answers: 2
You know the right answer?
Suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with iron(ii) c...
Questions

Chemistry, 13.10.2020 16:01


Business, 13.10.2020 16:01



English, 13.10.2020 16:01



Mathematics, 13.10.2020 16:01




Biology, 13.10.2020 16:01

History, 13.10.2020 16:01

Physics, 13.10.2020 16:01

Mathematics, 13.10.2020 16:01


Mathematics, 13.10.2020 16:01

Mathematics, 13.10.2020 16:01

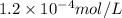 .
.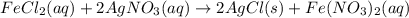
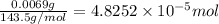
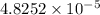 moles of silver chloride will be obtained from:
moles of silver chloride will be obtained from: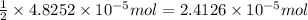
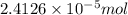
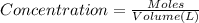
![[FeCl_2]=\frac{2.4126\times 10^{-5} mol}{0.2 L}=0.00012063 mol/L](/tpl/images/0173/9764/69c0f.png)
![[FeCl_2]=1.2063\times 10^{-4} mol/L\approx 1.2\times 10^{-4} mol/L](/tpl/images/0173/9764/77e47.png)


