
Chemistry, 10.08.2019 01:20 mikemurray115
The molar concentrations for the reactants and products at equilibrium are found to be [ccl4]=1.0 m, [o2]=0.3 m, [cocl2]=4.0 m, and [cl2]=2.0 m. what is the value of the equilibrium constant for this reaction? 2ccl4(g)+o2(g)⇌2cocl2(g)+2cl2(g) express your answer numerically using two significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
The molar concentrations for the reactants and products at equilibrium are found to be [ccl4]=1.0 m,...
Questions


History, 31.03.2021 19:50


Mathematics, 31.03.2021 19:50

Mathematics, 31.03.2021 19:50

Mathematics, 31.03.2021 19:50




Mathematics, 31.03.2021 19:50






Mathematics, 31.03.2021 19:50




 is the value of the equilibrium constant for this reaction.
is the value of the equilibrium constant for this reaction.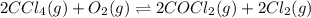
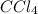 at equilibrium =
at equilibrium =![[CCl_4]=1.0M](/tpl/images/0174/0028/d343b.png)
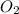 at equilibrium =
at equilibrium =![[O_2]=0.3M](/tpl/images/0174/0028/056b2.png)
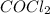 at equilibrium =
at equilibrium =![[COCl_2]=4.0M](/tpl/images/0174/0028/945ea.png)
 at equilibrium =
at equilibrium =![[Cl_2]=2.0M](/tpl/images/0174/0028/565ad.png)
![K_c=\frac{[COCl_2]^2[Cl_2]^2}{[CCl_4]^2[O_2]}=\frac{(4.0 M)^2\times (2.0M)^2}{(1.0M)^2\times (0.3 M)}](/tpl/images/0174/0028/1316e.png)



