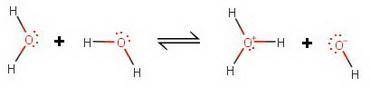Chemistry, 10.08.2019 01:30 rickevaaaa
Complete the equation for the ionization of water by drawing the conjugate acid and conjugate base. include lone pairs of electrons. two water molecules react to form the conjugate acid which has a plus 1 charge, and the conjugate base, which has a minus one charge. each water molecule consists of a central oxygen atom bonded to two hydrogen atoms. there are two lone pairs on the oxygen atom.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
Complete the equation for the ionization of water by drawing the conjugate acid and conjugate base....
Questions

Mathematics, 21.06.2021 01:40

Mathematics, 21.06.2021 01:40


Mathematics, 21.06.2021 01:40

Mathematics, 21.06.2021 01:40

English, 21.06.2021 01:40

French, 21.06.2021 01:40






Biology, 21.06.2021 01:40



Mathematics, 21.06.2021 01:40




English, 21.06.2021 01:40