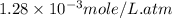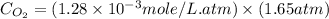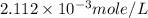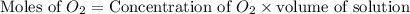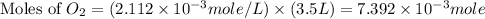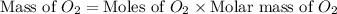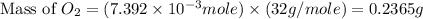Chemistry, 10.08.2019 03:10 lisacarter0804
The henry's law constant (kh) for o2 in water at 20°c is 1.28e-3 mol/l atm. how many grams of o2 will dissolve in 3.5 l of h2o that is in contact with pure o2 at 1.65 atm?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
The henry's law constant (kh) for o2 in water at 20°c is 1.28e-3 mol/l atm. how many grams of o2 wil...
Questions

History, 27.09.2019 21:40

English, 27.09.2019 21:40


Mathematics, 27.09.2019 21:40

Mathematics, 27.09.2019 21:40




Mathematics, 27.09.2019 21:40



Geography, 27.09.2019 21:40



Mathematics, 27.09.2019 21:40

Mathematics, 27.09.2019 21:40

Chemistry, 27.09.2019 21:40


History, 27.09.2019 21:40

Chemistry, 27.09.2019 21:40

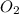 dissolved will be, 0.2365 grams
dissolved will be, 0.2365 grams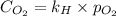
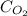 = concentration of
= concentration of 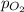 = partial pressure of
= partial pressure of 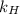 = Henry's law constant =
= Henry's law constant =