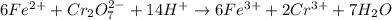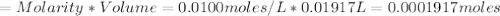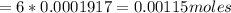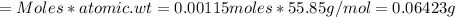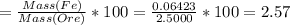Iron(ii) can be oxidized to iron(iii) by dichromate ion, which is reduced to chromium(iii) in acid solution. a 2.5000-g sample of iron ore is dissolved and the iron converted into iron(ii). exactly 19.17 ml of 0.0100 m na2cr2o7 is required in the titration. what percentage of the ore sample was iron?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3


Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Iron(ii) can be oxidized to iron(iii) by dichromate ion, which is reduced to chromium(iii) in acid s...
Questions


Mathematics, 31.07.2019 08:30


Mathematics, 31.07.2019 08:30



Spanish, 31.07.2019 08:30


Mathematics, 31.07.2019 08:30






Mathematics, 31.07.2019 08:30

Mathematics, 31.07.2019 08:30

Mathematics, 31.07.2019 08:30

History, 31.07.2019 08:30