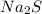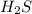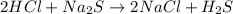For questions 22 – 24, write an equation for the reaction of hydrogen chloride and sodium sulfide to produce hydrogen sulfide with sodium chloride. (page 582 and page 567-568)
22. show the formulas of the reactants.
23. show the formulas of the products.
24. write the balanced the equation for this reaction. (page 585-587)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1


Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
You know the right answer?
For questions 22 – 24, write an equation for the reaction of hydrogen chloride and sodium sulfide to...
Questions




Arts, 28.10.2020 04:20


Biology, 28.10.2020 04:20

Mathematics, 28.10.2020 04:20


Mathematics, 28.10.2020 04:20

Computers and Technology, 28.10.2020 04:20

Spanish, 28.10.2020 04:20

Mathematics, 28.10.2020 04:20



Mathematics, 28.10.2020 04:20


English, 28.10.2020 04:20

Mathematics, 28.10.2020 04:20

History, 28.10.2020 04:20

SAT, 28.10.2020 04:20