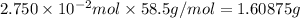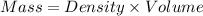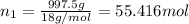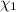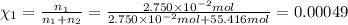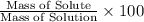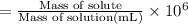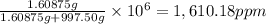Chemistry, 12.08.2019 16:10 lyndamahe0
A2.750×10−2m solution of nacl in water is at 20.0∘c. the sample was created by dissolving a sample of nacl in water and then bringing the volume up to 1.000 l. it was determined that the volume of water needed to do this was 999.3 ml . the density of water at 20.0∘c is 0.9982 g/ml.
calculate the mole fraction of salt in this solution.
calculate the concentration of the salt solution in percent by mass.
calculate the concentration of the salt solution in parts per million.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1
You know the right answer?
A2.750×10−2m solution of nacl in water is at 20.0∘c. the sample was created by dissolving a sample o...
Questions

Social Studies, 23.05.2021 23:40

Mathematics, 23.05.2021 23:40

Arts, 23.05.2021 23:40


Advanced Placement (AP), 23.05.2021 23:40






Mathematics, 23.05.2021 23:50


Mathematics, 23.05.2021 23:50

Mathematics, 23.05.2021 23:50



Mathematics, 23.05.2021 23:50

Computers and Technology, 23.05.2021 23:50


English, 23.05.2021 23:50


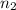
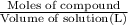
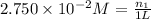
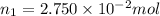
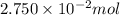 of NaCl :
of NaCl :