
Chemistry, 12.08.2019 18:30 briarkaltvedt
Calculate δ h° for the reaction c 4h 4( g) + 2h 2( g) → c 4h 8( g), using the following data: δ h° combustion for c 4h 4( g) = –2341 kj/mol δ h° combustion for h 2( g) = –286 kj/mol δ h° combustion for c 4h 8( g) = –2755 kj/mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1


Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
Calculate δ h° for the reaction c 4h 4( g) + 2h 2( g) → c 4h 8( g), using the following data: δ h°...
Questions

Social Studies, 19.09.2019 07:00

English, 19.09.2019 07:00

Biology, 19.09.2019 07:10


Biology, 19.09.2019 07:10


Mathematics, 19.09.2019 07:10




Mathematics, 19.09.2019 07:10

English, 19.09.2019 07:10








History, 19.09.2019 07:10

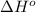
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_{(product)}]-\sum [n\times \Delta H^o_{(reactant)}]](/tpl/images/0174/3643/6872e.png)
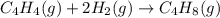
![\Delta H^o_{rxn}=[(1\times \Delta H^o_{(C_4H_8(g))})]-[(1\times \Delta H^o_{(C_4H_4(g))})+(2\times \Delta H^o_{(H_2(g))})]](/tpl/images/0174/3643/605f9.png)
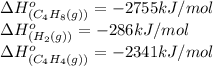
![\Delta H^o_{rxn}=[(1\times (-2755))]-[(1\times (-286))+(2\times (-2341))]\\\\\Delta H^o_{rxn}=2213kJ](/tpl/images/0174/3643/044fa.png)


