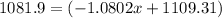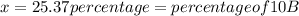The average atomic masses of some elements may vary, depending upon souces of their ores. naturally occurring boron consist of two isotopes with accurately known masses (10 b, 10.0129 amu and 11b, 11.0931 amu). the actual atomic mass of boron can vary grom 10.807 to 10819, depending on whether the mineral source is from turkey or the united states. calculate the percent abundances leading to the two values of the average atomic masses of boron from these two countries.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3


Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
The average atomic masses of some elements may vary, depending upon souces of their ores. naturally...
Questions

English, 20.05.2020 11:59


Business, 20.05.2020 11:59


Mathematics, 20.05.2020 11:59

Mathematics, 20.05.2020 11:59







History, 20.05.2020 11:59



Mathematics, 20.05.2020 11:59




![\frac{[percentageofisotope(1)Xatomicmassofisotope(1)]+[percentageofisotope(2)Xatomicmassofisotope(2)}{100}](/tpl/images/0174/3972/b6fa0.png)
![10.807=\frac{[x(10.0129)]+[(100-x)11.0931]}{100}=\frac{10.0129x+1109.31-11.0931x}{100}](/tpl/images/0174/3972/4e3aa.png)
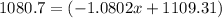
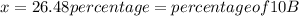
![10.819=\frac{[x(10.0129)]+[(100-x)11.0931]}{100}=\frac{10.0129x+1109.31-11.0931x}{100}](/tpl/images/0174/3972/7b5b5.png)