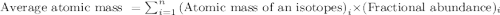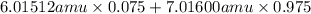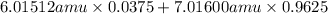Chemistry, 12.08.2019 19:20 kaywendel2008
Variations in average atomic mass may be observed for elements obtained from different sources. lithium provides an example of this. the isotopic composition of lithium from naturally occurring minerals is 7.5% 6li and 92.5% 7li, which have masses of 6.01512 amu and 7.01600 amu, respectively. a commercial source of lithium, recycled from a military source, was 3.75% 6li (and the rest 7li). calculate the average atomic mass values for each of these two sources.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
Variations in average atomic mass may be observed for elements obtained from different sources. lith...
Questions

Mathematics, 17.06.2020 20:57

Spanish, 17.06.2020 20:57

Mathematics, 17.06.2020 20:57


Mathematics, 17.06.2020 20:57


Mathematics, 17.06.2020 20:57

Mathematics, 17.06.2020 20:57

Mathematics, 17.06.2020 20:57

Mathematics, 17.06.2020 20:57


Social Studies, 17.06.2020 20:57

Mathematics, 17.06.2020 20:57

Chemistry, 17.06.2020 20:57


English, 17.06.2020 20:57

Mathematics, 17.06.2020 20:57