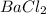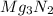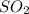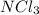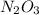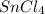Chemistry, 12.08.2019 20:10 ccarwile01
Write the formulas of the following compounds:
(a) barium chloride
(b) magnesium-nitride
(c) sulfur dioxide
(d) nitrogen trichloride
(e) dinitrogen trioxide
(f) tin(iv) chloride

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
Write the formulas of the following compounds:
(a) barium chloride
(b) magnesium-nitri...
(a) barium chloride
(b) magnesium-nitri...
Questions

Geography, 22.04.2021 01:00

Physics, 22.04.2021 01:00




Mathematics, 22.04.2021 01:00




Mathematics, 22.04.2021 01:00

History, 22.04.2021 01:00

English, 22.04.2021 01:00



Mathematics, 22.04.2021 01:00

Mathematics, 22.04.2021 01:00

Mathematics, 22.04.2021 01:00


Mathematics, 22.04.2021 01:00

History, 22.04.2021 01:00