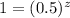Chemistry, 12.08.2019 20:30 ferguag15stu
The following data were obtained in a kinetics study of the hypothetical reaction a + b + c → products. [a]0 (m) [b]0 (m) [c]0 (m) initial rate (10–3 m/s) 0.4 0.4 0.2 160 0.2 0.4 0.4 80 0.6 0.1 0.2 15 0.2 0.1 0.2 5 0.2 0.2 0.4 20 using the initial-rate method, what is the order of the reaction with respect to c? a. zero-order b. first-order c. third-order d. second-order e. impossible to tell from the data given

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1


Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
The following data were obtained in a kinetics study of the hypothetical reaction a + b + c → produc...
Questions

Mathematics, 22.09.2021 21:40




Mathematics, 22.09.2021 21:40


Mathematics, 22.09.2021 21:40



Mathematics, 22.09.2021 21:40

Social Studies, 22.09.2021 21:40




English, 22.09.2021 21:40

History, 22.09.2021 21:40

Mathematics, 22.09.2021 21:40

Chemistry, 22.09.2021 21:40

Mathematics, 22.09.2021 21:40

![Rate = k[A]^{x}[B]^{y}[C]^{z}](/tpl/images/0174/4444/51b37.png)
![\frac{Rate3}{Rate4}= [\frac{[A(3)]}{[A(4)]}]^{x}](/tpl/images/0174/4444/34c33.png)
![\frac{15}{5}= [\frac{[0.6]}{[0.2]}]^{x}](/tpl/images/0174/4444/c782c.png)
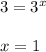
![\frac{Rate2}{Rate5}= [\frac{[B(2)]}{[B(5)]}]^{y}](/tpl/images/0174/4444/f0ddb.png)
![\frac{80}{20}= [\frac{[0.4]}{[0.2]}]^{y}](/tpl/images/0174/4444/8ed1b.png)
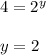
![\frac{Rate1}{Rate2}= [\frac{[A(1)]}{[A(2)]}]^{x}[\frac{[B(1)]}{[B(2)]}]^{y}[\frac{[C(1)]}{[C(2)]}]^{z}](/tpl/images/0174/4444/66487.png)
![\frac{160}{80}= [\frac{[0.4]}{[0.2]}]^{1}[\frac{[0.4]}{[0.4]}]^{2}[\frac{[0.2]}{[0.4]}]^{z}](/tpl/images/0174/4444/7ba82.png)