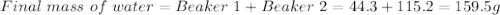Chemistry, 12.08.2019 21:20 hannahkharel2
Astudent pours 44.3 g of water at 10â°c into a beaker containing 115.2 g of water at 10â°c. what are the final mass, temperature, and density of the combined water? the density of water at 10â°c is 1.00 g/ml.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 14:00
Cassandra made a venn diagram to compare and contrast the two stages of cellular respiration. which belongs in the area marked x? energy is released. oxygen is used up. glucose is broken down. carbon dioxide is used up.
Answers: 1

You know the right answer?
Astudent pours 44.3 g of water at 10â°c into a beaker containing 115.2 g of water at 10â°c. what are...
Questions

History, 21.07.2019 19:30

Physics, 21.07.2019 19:30


Computers and Technology, 21.07.2019 19:30

Mathematics, 21.07.2019 19:30


Health, 21.07.2019 19:30

History, 21.07.2019 19:30

Mathematics, 21.07.2019 19:30

History, 21.07.2019 19:30

Biology, 21.07.2019 19:30





Biology, 21.07.2019 19:30


History, 21.07.2019 19:30