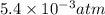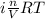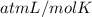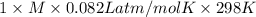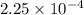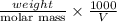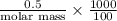Chemistry, 12.08.2019 22:20 cravingnafi202
Asolution of 0.5 g of an unknown nonvolatile, nonelectrolyte solute is added to 100 ml of water and then placed across a semipermeable membrane from a volume of pure water. when the system reaches equilibrium, the solution compartment is elevated 5.6 cm above the solvent compartment. assuming that the density of the solution is 1.0 g / ml, calculate the molecular mass of the unknown.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Asolution of 0.5 g of an unknown nonvolatile, nonelectrolyte solute is added to 100 ml of water and...
Questions

Mathematics, 04.12.2019 06:31

Mathematics, 04.12.2019 06:31







Mathematics, 04.12.2019 06:31




English, 04.12.2019 06:31



Mathematics, 04.12.2019 06:31


Biology, 04.12.2019 06:31


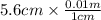 = 0.056, density = 1.0 g/ml, g = 9.8 m/s
= 0.056, density = 1.0 g/ml, g = 9.8 m/s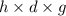
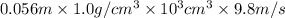
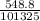 atm
atm