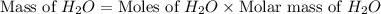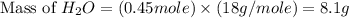Chemistry, 12.08.2019 23:30 nmartin5185
If 50.0 dm3 of methane, ch4, react with 10.0 dm3 of air, calculate the grams of water produced.
ch4 (g) + 2 o2 (g) --> co2 (g) + 2 h2o (l)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
If 50.0 dm3 of methane, ch4, react with 10.0 dm3 of air, calculate the grams of water produced.
Questions

English, 15.01.2021 23:10


History, 15.01.2021 23:10


Health, 15.01.2021 23:10


Biology, 15.01.2021 23:10


Mathematics, 15.01.2021 23:10


Mathematics, 15.01.2021 23:10


History, 15.01.2021 23:10

History, 15.01.2021 23:10

Mathematics, 15.01.2021 23:10

Advanced Placement (AP), 15.01.2021 23:10

Health, 15.01.2021 23:10


Mathematics, 15.01.2021 23:10

Advanced Placement (AP), 15.01.2021 23:10

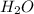 produced will be, 8.1 grams.
produced will be, 8.1 grams.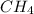 =
= 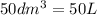
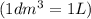
 =
= 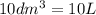
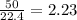 mole of
mole of 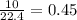 mole of
mole of 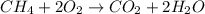
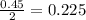 moles of
moles of