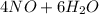Chemistry, 13.08.2019 00:30 calvinsanchez8429
Gaseous ammonia chemically reacts with oxygen gas to produce nitrogen monoxide gas and water vapor. calculate the moles of oxygen needed to produce of water. be sure your answer has a unit symbol, if necessary, and round it to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
Gaseous ammonia chemically reacts with oxygen gas to produce nitrogen monoxide gas and water vapor....
Questions

Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00



English, 11.12.2021 01:00

Spanish, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00

Chemistry, 11.12.2021 01:00


English, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00


Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00


Arts, 11.12.2021 01:00

Business, 11.12.2021 01:00

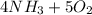 ⇒
⇒