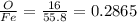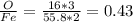Chemistry, 13.08.2019 01:30 katwright1124
Both feo and fe2o3 contain only iron and oxygen. the mass ratio of oxygen to iron for each compound is given in the following table:
compound mass o : mass fe
feo 0.2865
fe2o3 0.4297
show that these data are consistent with the law of multiple proportions.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

You know the right answer?
Both feo and fe2o3 contain only iron and oxygen. the mass ratio of oxygen to iron for each compound...
Questions




Mathematics, 04.04.2020 23:17

Mathematics, 04.04.2020 23:17


Mathematics, 04.04.2020 23:18


Mathematics, 04.04.2020 23:18



Mathematics, 04.04.2020 23:18

Mathematics, 04.04.2020 23:18


English, 04.04.2020 23:18

Mathematics, 04.04.2020 23:18

Social Studies, 04.04.2020 23:18

Chemistry, 04.04.2020 23:18

Advanced Placement (AP), 04.04.2020 23:18