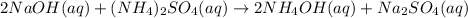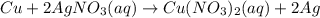Chemistry, 13.08.2019 01:30 Rockey3876
Which statement is correct about the reaction that occurs between copper and aqueous silver nitrate?
it is a single replacement reaction because two new compounds of copper and silver are formed.
it is a double replacement reaction because copper and silver exchange their non-metallic parts.
it is a single replacement reaction because copper replaces silver within a compound as copper is higher than silver in the activity series.
it is a double replacement reaction because the ions of copper and silver exchange places in an aqueous solution as copper is higher than silver in the activity series.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2


Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
Which statement is correct about the reaction that occurs between copper and aqueous silver nitrate?...
Questions



Biology, 18.09.2021 14:00


Mathematics, 18.09.2021 14:00



English, 18.09.2021 14:00


History, 18.09.2021 14:00

Mathematics, 18.09.2021 14:00

Chemistry, 18.09.2021 14:00

Mathematics, 18.09.2021 14:00

World Languages, 18.09.2021 14:00

Advanced Placement (AP), 18.09.2021 14:00

Spanish, 18.09.2021 14:00

Mathematics, 18.09.2021 14:00



Business, 18.09.2021 14:00