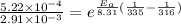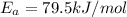Chemistry, 13.08.2019 02:20 dramaqueenactr2040
The rate constants for the first-order decomposition of a compound are 5.22× 10–4 s–1 at 43°c and 2.91 × 10–3 s–1 at 62°c. what is the value of the activation energy for this reaction? (r = 8.31 j/(mol · k)) a. 79.5 kj/mol b. 34.5 kj/mol c. 0.751 kj/mol d. 0.87104 kj/mol e. 2 kj/mol

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
The rate constants for the first-order decomposition of a compound are 5.22× 10–4 s–1 at 43°c and 2....
Questions

Mathematics, 04.12.2020 07:40


Business, 04.12.2020 07:40




Mathematics, 04.12.2020 07:40

Geography, 04.12.2020 07:40





Chemistry, 04.12.2020 07:40


Mathematics, 04.12.2020 07:40

Mathematics, 04.12.2020 07:40

History, 04.12.2020 07:40

Mathematics, 04.12.2020 07:40


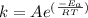
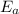 is the activation energy and T is temperature in kelvin
is the activation energy and T is temperature in kelvin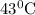 ,
, 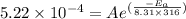 ............(1)
............(1)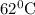 ,
, 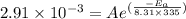 ............(2)
............(2)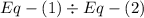 gives-
gives-