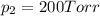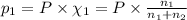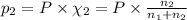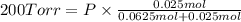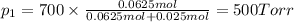Chemistry, 13.08.2019 02:30 lailahussain99
Agas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40.0). if the partial pressure of argon is 200. torr, what is the pressure of methane, in torr? hint: what is the mole fraction of each gas?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1


Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
Agas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40...
Questions

History, 25.01.2021 23:30

Mathematics, 25.01.2021 23:30


Advanced Placement (AP), 25.01.2021 23:30


Mathematics, 25.01.2021 23:30

Medicine, 25.01.2021 23:30

History, 25.01.2021 23:30


English, 25.01.2021 23:30


Mathematics, 25.01.2021 23:30

History, 25.01.2021 23:30

Mathematics, 25.01.2021 23:30


Physics, 25.01.2021 23:30

Biology, 25.01.2021 23:30

Mathematics, 25.01.2021 23:30


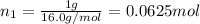
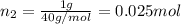
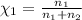
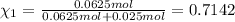
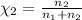
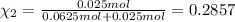
 .
.