
Which of the following oxidizing agents will oxidize br– but not cl–? 2br– ⇔ br2(l) + 2e– e°ox = –1.065 v 2cl– ⇔ cl2(g) + 2e– e°ox = –1.359 v cr2o72–(aq) + 14h+(aq) + 6e– t h i s a n s w e r i s s e t a s c o r r e c t 7h2o(l) + 2cr3+(aq) e°red =+1.33 v mno4–(aq) + 8h+(aq) + 5e– ⇔ mn2+(aq) + 4h2o(l) e°red = +1.51v no3–(aq) + 4h+(aq) + 3e– ⇔ no(g) + 2h2o(l) e°red =+0.96 v

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

You know the right answer?
Which of the following oxidizing agents will oxidize br– but not cl–? 2br– ⇔ br2(l) + 2e– e°ox = –1...
Questions





Social Studies, 16.10.2019 22:30


History, 16.10.2019 22:30

Social Studies, 16.10.2019 22:30

Mathematics, 16.10.2019 22:30

Mathematics, 16.10.2019 22:30



Mathematics, 16.10.2019 22:30





Mathematics, 16.10.2019 22:30

Physics, 16.10.2019 22:30

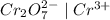 system oxidizes
system oxidizes 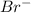 but not
but not 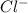 .
.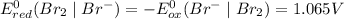
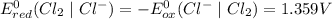
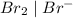 system but have lesser standard reduction potential than
system but have lesser standard reduction potential than 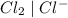 system will oxidize
system will oxidize 


