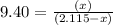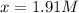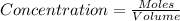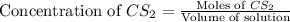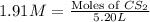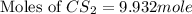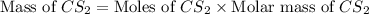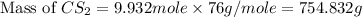Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2


Chemistry, 23.06.2019 22:30
3. a 0.750-l container containing gas at a temperature of 21.3º c is transferred to a flask with a volume of 0.250-l. assuming there is no change in pressure during or after the transfer, what is the temperature of the gas in the new flask?
Answers: 1
You know the right answer?
S2 + c > cs2how many grams of cs2 can be prepared by heating 11.0 moles of s2 with excess carbon...
Questions

Chemistry, 16.07.2021 08:00

Mathematics, 16.07.2021 08:00


Health, 16.07.2021 08:00

Mathematics, 16.07.2021 08:00

Mathematics, 16.07.2021 08:00



History, 16.07.2021 08:00


English, 16.07.2021 08:00

Biology, 16.07.2021 08:00


Mathematics, 16.07.2021 08:10

Mathematics, 16.07.2021 08:10


Physics, 16.07.2021 08:10

Health, 16.07.2021 08:10

Mathematics, 16.07.2021 08:10

English, 16.07.2021 08:10

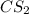 prepared can be, 754.832 grams
prepared can be, 754.832 grams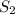 = 11.0 mole
= 11.0 mole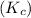 = 9.40
= 9.40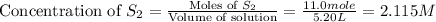
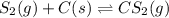
![K_c=\frac{[CS_2]}{[S_2]}](/tpl/images/0174/7128/0a94f.png)