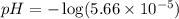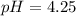Chemistry, 13.08.2019 04:30 jenifferplowman
The acid dissociation ka of propionic acid c2h5co2h is 1.310x10−5. calculate the ph of a 3.010x10−4m aqueous solution of propionic acid. round your answer to 2 decimal places.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
The acid dissociation ka of propionic acid c2h5co2h is 1.310x10−5. calculate the ph of a 3.010x10−4m...
Questions



Business, 01.07.2020 15:01



English, 01.07.2020 15:01

History, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01

English, 01.07.2020 15:01

English, 01.07.2020 15:01



Computers and Technology, 01.07.2020 15:01

Social Studies, 01.07.2020 15:01




Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01

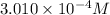
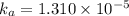
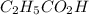 (weak acid) is,
(weak acid) is,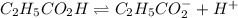
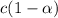
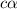
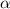 is degree of dissociation
is degree of dissociation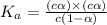
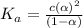
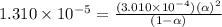
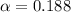
![[H^+]=c\alpha=(3.010\times 10^{-4})\times (0.188)=5.66\times 10^{-5}M](/tpl/images/0174/7681/497f7.png)
![pH=-\log [H^+]](/tpl/images/0174/7681/37e81.png)