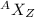Chemistry, 13.08.2019 04:30 nataliellamas56
For the noble gases (the group 8a elements) 4he2, 20ne10, 40ar18, 84kr36 and 132xe54, (a) determine the number of protons and neutrons in the nucleus of each atom, and (b) determine the ratio of neutrons to protons in the nucleus of each atom. describe any general trend you discover in the way this ratio changes with increasing atomic number.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

You know the right answer?
For the noble gases (the group 8a elements) 4he2, 20ne10, 40ar18, 84kr36 and 132xe54, (a) determine...
Questions

Social Studies, 29.01.2020 03:54


English, 29.01.2020 03:54


Social Studies, 29.01.2020 03:54

Mathematics, 29.01.2020 03:54



Mathematics, 29.01.2020 03:54



Mathematics, 29.01.2020 03:54



Biology, 29.01.2020 03:54

Mathematics, 29.01.2020 03:54

English, 29.01.2020 03:54

Mathematics, 29.01.2020 03:54

Physics, 29.01.2020 03:54

Mathematics, 29.01.2020 03:54