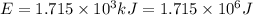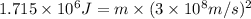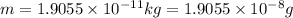In the second footnote it was pointed out that mass and energy are alternate aspects of a single entity called mass-energy. the relationship between these two physical quantities is einstein's equation, e= mc^2, where e is energy, m is mass, and c is the speed of light. in a combustion experiment, it was found that 12.096 g of hydrogen molecules combined with 96.000 g of oxygen molecules to form water and released 1.715 x 10^3 kj of heat. use einstein's equation to calculate the corresponding mass change in this process, and comment on whether or not the law of conservation of mass holds for ordinary chemical processes.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
In the second footnote it was pointed out that mass and energy are alternate aspects of a single ent...
Questions



Mathematics, 28.04.2021 16:50

Biology, 28.04.2021 16:50

Computers and Technology, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50




Mathematics, 28.04.2021 16:50

Biology, 28.04.2021 16:50



English, 28.04.2021 16:50



Chemistry, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Physics, 28.04.2021 16:50

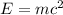 ,
,