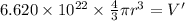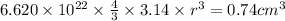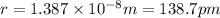Chemistry, 13.08.2019 05:10 gachaperson123
Acube made of platinum (pt) has an edge length of 1.0 cm. (a) calculate the number of pt atoms in the cube. (b) atoms are spherical in shape. therefore, the pt atoms in the cube cannot fill all the available space. if only 74 percent of the space inside the cube is taken up by pt atoms, calculate the radius in picometers of a pt atom. the density pt is 21.45 g/cm3, and the mass of a single pt atom is 3.240 x 10^-22 g. (the volume of a sphere of radius r is 4/5ïr^3).

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Acube made of platinum (pt) has an edge length of 1.0 cm. (a) calculate the number of pt atoms in th...
Questions


Mathematics, 06.07.2019 23:00

English, 06.07.2019 23:00






History, 06.07.2019 23:00




English, 06.07.2019 23:00

Physics, 06.07.2019 23:00



Chemistry, 06.07.2019 23:00

English, 06.07.2019 23:00


Mathematics, 06.07.2019 23:00

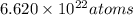 .
.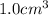
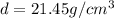
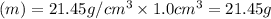
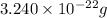
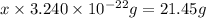
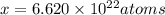
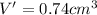
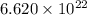 atoms is
atoms is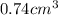 .
.