
Chemistry, 13.08.2019 05:10 natajaeecarr
Below is a proposed mechanism for the decomposition of h2o2. h2o2 + i– → h2o + io– slow h2o2 + io– → h2o + o2 + i– fast which of the following statements is incorrect? a. io– is a catalyst. b. the reaction is first-order with respect to [i–]. c. the reaction is first-order with respect to [h2o2]. d. the net reaction is 2h2o2 → 2h2o + o2. e. i– is a catalyst.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2


Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Below is a proposed mechanism for the decomposition of h2o2. h2o2 + i– → h2o + io– slow h2o2 + io– →...
Questions

Biology, 27.08.2019 09:10



Health, 27.08.2019 09:10






Mathematics, 27.08.2019 09:10

Social Studies, 27.08.2019 09:10

Mathematics, 27.08.2019 09:10




Biology, 27.08.2019 09:10

Arts, 27.08.2019 09:10

Mathematics, 27.08.2019 09:10

Mathematics, 27.08.2019 09:10

Health, 27.08.2019 09:10

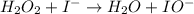 (slow)
(slow)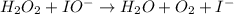
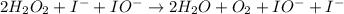
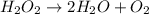
![k[H_{2}O_{2}][I^{-}]](/tpl/images/0174/7990/a32b4.png)
![[I^{-}]](/tpl/images/0174/7990/13772.png) and it is also first order reaction with respect to
and it is also first order reaction with respect to ![[H_{2}O_{2}]](/tpl/images/0174/7990/955b6.png) .
.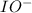 is a catalyst.
is a catalyst.

