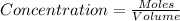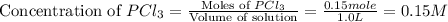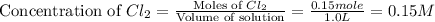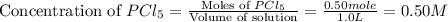Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Initially, 0.65 mol of pcl5 is placed in a 1.0 l flask. at equilibrium, there is 0.15 mol of pcl3 in...
Questions

Mathematics, 19.01.2020 04:31

Mathematics, 19.01.2020 04:31



Biology, 19.01.2020 04:31



History, 19.01.2020 04:31

Spanish, 19.01.2020 04:31







Mathematics, 19.01.2020 04:31


Physics, 19.01.2020 04:31

Mathematics, 19.01.2020 04:31

Mathematics, 19.01.2020 04:31

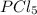 is, 0.50 M
is, 0.50 M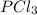 at equilibrium = 0.15 mole
at equilibrium = 0.15 mole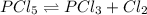
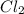 at equilibrium = x = 0.15 mole
at equilibrium = x = 0.15 mole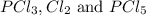 at equilibrium.
at equilibrium.