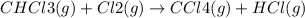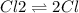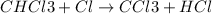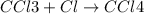Chemistry, 13.08.2019 05:10 meCreation
The reaction chcl3(g) + cl2(g) ccl4(g) + hcl(g) has been proposed to occur by the following mechanism. cl2 2cl fast equilibrium chcl3 + cl hcl + ccl3 slow step ccl3 + cl ccl4 fast what is the rate law predicted by this mechanism? a. rate = k2[chcl3][cl] b. rate = kexp[chcl3][cl2] c. rate = k2(k1/k-1)1/2[chcl3][cl2]1/2 d. rate = k3[ccl3][cl] e. rate = k1[cl2]

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2

Chemistry, 23.06.2019 12:30
0.070g of hydride of carbon occupies 56cm^3 at s.t.p when vaporized and contained 14.29% by mass of hydrogen.what is the formula for the hydrocarbon
Answers: 1
You know the right answer?
The reaction chcl3(g) + cl2(g) ccl4(g) + hcl(g) has been proposed to occur by the following mechanis...
Questions




Mathematics, 04.06.2021 02:10

Mathematics, 04.06.2021 02:10

Mathematics, 04.06.2021 02:10

English, 04.06.2021 02:10


Mathematics, 04.06.2021 02:10



Social Studies, 04.06.2021 02:10




Arts, 04.06.2021 02:10

Chemistry, 04.06.2021 02:10

Mathematics, 04.06.2021 02:10