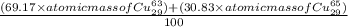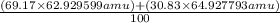Chemistry, 13.08.2019 05:20 johnsonkia873
The atomic masses of the two stable isotopes of copper 63cu29 (69.17 percent) and 65cu29 (30.83 percent)â are 62.929599 and 64.927793 amu, respectively. calculate the average atomic mass of copper.

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1
You know the right answer?
The atomic masses of the two stable isotopes of copper 63cu29 (69.17 percent) and 65cu29 (30.83 perc...
Questions

Mathematics, 19.05.2021 03:30

Mathematics, 19.05.2021 03:30

Biology, 19.05.2021 03:30

Mathematics, 19.05.2021 03:30






Spanish, 19.05.2021 03:30




Mathematics, 19.05.2021 03:30

History, 19.05.2021 03:30

History, 19.05.2021 03:30

SAT, 19.05.2021 03:30

Social Studies, 19.05.2021 03:30


Mathematics, 19.05.2021 03:30

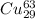 atoms and 30.83 number of
atoms and 30.83 number of 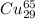 atoms.
atoms.