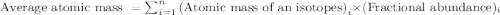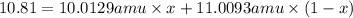Chemistry, 13.08.2019 05:30 luclaymom805
Boron has two naturally occurring isotopes, 10b and 11b, which have masses 10.0129 and 11.0093 amu, respectively. given the average atomic mass of boron (10.81 amu), determine the percent abundance of each isotope. (a) 50%10b, 50%11b (b) 20%10b, 80%11b (c) 98%10b, 2% 11b (d) 93%10b, 7%11b (e) 22%10b, 78%11b

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1


Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1
You know the right answer?
Boron has two naturally occurring isotopes, 10b and 11b, which have masses 10.0129 and 11.0093 amu,...
Questions


Mathematics, 18.10.2020 16:01


English, 18.10.2020 16:01






English, 18.10.2020 16:01

Chemistry, 18.10.2020 16:01



Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01


Mathematics, 18.10.2020 16:01



Spanish, 18.10.2020 16:01