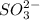Chemistry, 13.08.2019 05:30 naysia2006
Deduce the formulas of the following ionic compounds: (a) iron(lll) sulfide, (b) mercury(ll) nitrate, and (c) potassium sulfite.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1


Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Deduce the formulas of the following ionic compounds: (a) iron(lll) sulfide, (b) mercury(ll) nitrat...
Questions




Biology, 24.11.2019 05:31




Mathematics, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31




Arts, 24.11.2019 05:31

Geography, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31


Geography, 24.11.2019 05:31

World Languages, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31

History, 24.11.2019 05:31

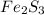
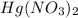
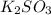
![[Ar]3d^64s^2](/tpl/images/0174/8186/7fd67.png) .
.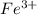 ion, this element will loose 3 electrons.
ion, this element will loose 3 electrons.![[Ne]3s^23p^4](/tpl/images/0174/8186/9a0fd.png) .
.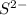 ion, this element will gain 2 electrons.
ion, this element will gain 2 electrons. ![[Xe]4f^{14}5d^{10}6s^2](/tpl/images/0174/8186/13e4d.png) .
.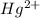 ion, this element will loose 2 electrons.
ion, this element will loose 2 electrons.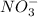
![[Ar]4s^1](/tpl/images/0174/8186/85db5.png) .
.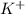 ion, this element will loose 1 electron.
ion, this element will loose 1 electron.