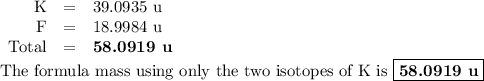Chemistry, 14.08.2019 08:20 petroale000
The two isotopes of potassium with significant abundance in nature are 39k asotopic mass 38.9637 amu, 932.58%) and 41k osotopic mass 40.9618 amu, 6.730%). fluorine has only one naturally occurring isotope, 19f (isotopic mass 18.9984 amu). calculate the formula mass of potassium fluoride.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
The two isotopes of potassium with significant abundance in nature are 39k asotopic mass 38.9637 amu,...
Questions



Mathematics, 22.05.2021 01:30


Mathematics, 22.05.2021 01:30


Mathematics, 22.05.2021 01:30

Mathematics, 22.05.2021 01:30


Mathematics, 22.05.2021 01:30

Mathematics, 22.05.2021 01:30

Physics, 22.05.2021 01:30

English, 22.05.2021 01:30



Mathematics, 22.05.2021 01:30

Computers and Technology, 22.05.2021 01:30

Chemistry, 22.05.2021 01:30


Mathematics, 22.05.2021 01:30