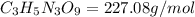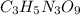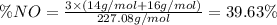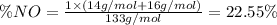Nitrogen monoxide (no) is a bioactive molecule in blood. low no concentrations cause respiratory distress and the formation of blood clots. doctors prescribe nitroglycerin, c3h5n 309. and isoamyl nitrate. (ch3)2chch2ch20n02, to increase no. if each compound releases one molecule of no per atom of n. calculate the mass percent of no in each medicine.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
Nitrogen monoxide (no) is a bioactive molecule in blood. low no concentrations cause respiratory dis...
Questions


Mathematics, 23.02.2021 03:00

Physics, 23.02.2021 03:00


Mathematics, 23.02.2021 03:00

Mathematics, 23.02.2021 03:00

Biology, 23.02.2021 03:00








Mathematics, 23.02.2021 03:00

Mathematics, 23.02.2021 03:00