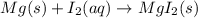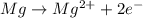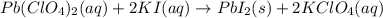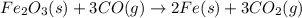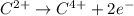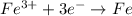Chemistry, 16.08.2019 08:10 avastanleyy
Which of the following chemical equations show(s) oxidation-reduction reactions? 1. mg(s) + i2(aq) --> mgi2(s)2. pb(clo4)2(aq) + 2 ki(aq) --> pbi2(s) + 2 kclo4(aq)3. fe2o3(s) + 3 co(g) --> 2 fe(s) + 3 co2(g)1 only2 only1 and 21 and 32 and 3

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1

You know the right answer?
Which of the following chemical equations show(s) oxidation-reduction reactions? 1. mg(s) + i2(aq) -...
Questions



English, 09.10.2021 04:30



Chemistry, 09.10.2021 04:30



History, 09.10.2021 04:30





History, 09.10.2021 04:30

Mathematics, 09.10.2021 04:30


Biology, 09.10.2021 04:30

Mathematics, 09.10.2021 04:30


Biology, 09.10.2021 04:30