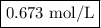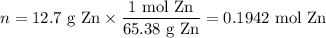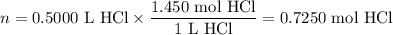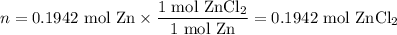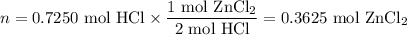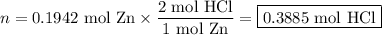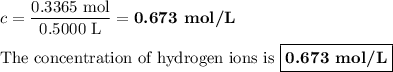Chemistry, 16.08.2019 08:20 JAYDENJONES0111
Zinc dissolves in hydrochloric acid to yield hydrogen gas: zn(s) + 2hcl(aq) --> zncl2(aq) + h2(g) when a 12.7 g chunk of zinc dissolves in 5.00 x 102 ml of 1.450 m hcl, what is the concentration of hydrogen ions remaining in the final solution? 0 m0.388 m0.674 m0.776 m1.06 m

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
You know the right answer?
Zinc dissolves in hydrochloric acid to yield hydrogen gas: zn(s) + 2hcl(aq) --> zncl2(aq) + h2(...
Questions

Mathematics, 01.01.2022 14:20


Mathematics, 01.01.2022 14:30


Mathematics, 01.01.2022 14:30

Computers and Technology, 01.01.2022 14:30



History, 01.01.2022 14:30

Mathematics, 01.01.2022 14:30

Mathematics, 01.01.2022 14:30





Mathematics, 01.01.2022 14:40



Mathematics, 01.01.2022 14:40

History, 01.01.2022 14:40