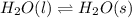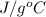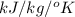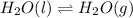Question 2: phase changes (14 points) a. rewrite each of the following equations for phase changes, to include the heat required for the phase change. use values from latent heat and specific heat constant tables when necessary. indicate whether each phase change is endothermic or exothermic. (2 points) i. h2o(l) h2o(s) (1 point) ii. h2o(l) h2o(g) (1 point)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
Question 2: phase changes (14 points) a. rewrite each of the following equations for phase changes,...
Questions






English, 12.08.2020 09:01



Mathematics, 12.08.2020 09:01




Arts, 12.08.2020 09:01

Health, 12.08.2020 09:01


Mathematics, 12.08.2020 09:01


Biology, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01