
Chemistry, 17.08.2019 19:10 brittanyfox411
A25.00-ml sample of propionic acid, hc3h5o2, of unknown concentration was titrated with 0.141 m koh. the equivalence point was reached when 43.76 ml of base had been added. what is the hydroxide-ion concentration at the equivalence point? ka for propionic acid is 1.3 × 10–5 at 25°c. a. 1.5 × 10-9 m b. 1.1 × 10-3 m c. 1.1 × 10-5 m d. 8.3 × 10-6 m e. 1.0 × 10-7 m

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
A25.00-ml sample of propionic acid, hc3h5o2, of unknown concentration was titrated with 0.141 m koh....
Questions

History, 31.07.2019 22:50


History, 31.07.2019 22:50

Biology, 31.07.2019 22:50

Biology, 31.07.2019 22:50



Mathematics, 31.07.2019 22:50


Social Studies, 31.07.2019 22:50


Biology, 31.07.2019 22:50


Mathematics, 31.07.2019 22:50

Social Studies, 31.07.2019 22:50


Mathematics, 31.07.2019 22:50

Biology, 31.07.2019 22:50

Biology, 31.07.2019 22:50

History, 31.07.2019 22:50

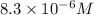
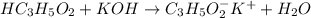
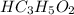 reacts with 1 mol of KOH to produce 1 mol of
reacts with 1 mol of KOH to produce 1 mol of 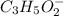
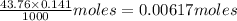
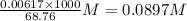
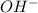 produced at equivalence point is due to hydrolysis of
produced at equivalence point is due to hydrolysis of 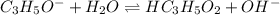
![\frac{[HC_{3}H_{5}O_{2}][OH^{-}]}{[C_{3}H_{5}O_{2}^{-}]}=K_{b}(C_{3}H_{5}O_{2}^{-})=\frac{10^{-14}}{K_{a}(HC_{3}H_{5}O_{2})}](/tpl/images/0175/3912/dc120.png)
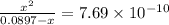
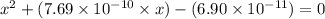
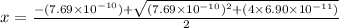 M =
M = 

