
Chemistry, 18.08.2019 00:10 homelanie21
Acell was set up having the following reaction mg(s) + cd2+ (aq) → mg2+ (aq) + cd (s) e°cell = 1.97 v the magnesium electrode was dipped in a 1.00 m solution of mgso4 and the cadmium electrode was dipped in a solution of unknown cd2+ concentration. the cell potential was measured to be 1.80 v. what is the unknown cd2+ concentration?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

You know the right answer?
Acell was set up having the following reaction mg(s) + cd2+ (aq) → mg2+ (aq) + cd (s) e°cell = 1.97...
Questions

Mathematics, 07.12.2020 03:10




English, 07.12.2020 03:10



Mathematics, 07.12.2020 03:10

English, 07.12.2020 03:10

History, 07.12.2020 03:10


Health, 07.12.2020 03:10

Mathematics, 07.12.2020 03:10

Advanced Placement (AP), 07.12.2020 03:10

Mathematics, 07.12.2020 03:10



Mathematics, 07.12.2020 03:10


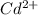 will be,
will be, 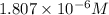
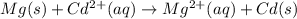
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Mg^{2+}]}{[Cd^{2+}]}](/tpl/images/0175/4823/30e69.png)
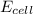 = emf of the cell = 1.80 V
= emf of the cell = 1.80 V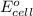 = standard cell potential = 1.97 V
= standard cell potential = 1.97 V![[Mg^{2+}]](/tpl/images/0175/4823/9cb7c.png) = concentration of magnesium ion = 1.00 M
= concentration of magnesium ion = 1.00 M![[Cd^{2+}]](/tpl/images/0175/4823/f7ff3.png) = concentration of cadmium ion = ?
= concentration of cadmium ion = ?![1.80=1.97-\frac{0.0592}{2}\log \frac{(1.00)}{[Cd^{2+}]}](/tpl/images/0175/4823/3b1a8.png)
![[Cd^{2+}]=1.807\times 10^{-6}M](/tpl/images/0175/4823/bd39b.png)


