
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 1.74 g of butane is mixed with 11. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions



Health, 11.11.2020 17:30

English, 11.11.2020 17:30

Mathematics, 11.11.2020 17:30

English, 11.11.2020 17:30

History, 11.11.2020 17:30

Mathematics, 11.11.2020 17:30

Mathematics, 11.11.2020 17:30





Mathematics, 11.11.2020 17:30




Chemistry, 11.11.2020 17:30


Mathematics, 11.11.2020 17:30

 produced will be, 5.3 grams.
produced will be, 5.3 grams. = 1.74 g
= 1.74 g = 11 g
= 11 g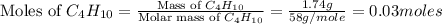


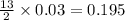 moles of
moles of  moles of
moles of 



