
Chloral hydrate (c2h3cl3o2) is a drug formerly used as a sedative and hypnotic.
a. calculate the molar mass of chloral hydrate. b. what amount (moles) of c2h3cl3o2 molecules are in 500.0 g chloral hydrate? c. what is the mass in grams of 2.0 x 10-2 mol chloral hydrate? d. what number of chlorine atoms are in 5.0 g chloral hydrate? e. what mass of chloral hydrate would contain 1.0 g cl? f. what is the mass of exactly 500 molecules of chloral hydrate?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

You know the right answer?
Chloral hydrate (c2h3cl3o2) is a drug formerly used as a sedative and hypnotic.
a. calculate...
a. calculate...
Questions

Mathematics, 06.02.2021 05:40

History, 06.02.2021 05:40

Computers and Technology, 06.02.2021 05:40


Advanced Placement (AP), 06.02.2021 05:40


English, 06.02.2021 05:40




Chemistry, 06.02.2021 05:40




Health, 06.02.2021 05:40


Mathematics, 06.02.2021 05:40


Advanced Placement (AP), 06.02.2021 05:40

 is, 165.5 g/mole
is, 165.5 g/mole mole chloral hydrate is, 3.31 g
mole chloral hydrate is, 3.31 g

 =2(12g/mole)+3(1g/mole)+3(35.5g/mole)+2(16g/mole)=165.5g/mole[/tex]
=2(12g/mole)+3(1g/mole)+3(35.5g/mole)+2(16g/mole)=165.5g/mole[/tex]



 chlorine atoms
chlorine atoms chlorine atoms
chlorine atoms of chlorine present in 165.5 g of
of chlorine present in 165.5 g of  of
of  molecules of chloral hydrate has 165.5 g mass of chloral hydrate
molecules of chloral hydrate has 165.5 g mass of chloral hydrate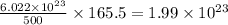 mass of chloral hydrate
mass of chloral hydrate

