
Chemistry, 18.08.2019 04:10 kadence428
The cell potential of the following electrochemical cell depends on the gold concentration in the cathode half-cell: pt(s)|h2(g,1atm)|h+(aq,1.0m)|au3+(a q,? m)|au(s). what is the concentration of au3+ in the solution if ecell is 1.27 v ? express your answer using two significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2


Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
The cell potential of the following electrochemical cell depends on the gold concentration in the ca...
Questions

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

English, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

English, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 14:01

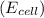 is given as 1.27 V.
is given as 1.27 V.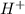 = 1.0 M
= 1.0 M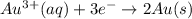
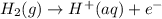
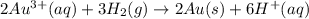
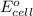 ) for hydrogen is equal to zero.
) for hydrogen is equal to zero.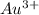 is
is  equal 1.50 V.
equal 1.50 V.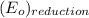 -
- 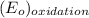
![E_{cell} = E^{o}_{cell} - \frac{0.059}{2}log \frac{[H^{+}]^{6}}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/b4490.png)
![log\frac{1}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/7ec17.png) = 23
= 23![\frac{1}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/cebd5.png) =
= 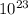
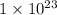
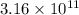
![[Au^{3+}]](/tpl/images/0175/5466/7b483.png) =
= 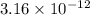 M
M

